Aligned for Success
Patients win when sites, sponsors and monitors work together.
Clinical Trial Sponsors
Meet Study Goals Faster as Sponsor of Choice
Sponsors gain actionable insights into trial conduct to address risk and meet your study goals proactively. Break down traditional communication barriers. Provide timely, relevant guidance and expert support directly to sites.

Stay Top of Mind
Engage sites with targeted messages to the right people at the right time. Message templates and instant access to study staff contact lists reduce time and effort to communicate.
Collaborate at Speed
Digital. Mobile. At the point of care. Interconnected teams deliver site support through a secure, dedicated channel for high-fidelity answers in seconds – not hours or days later.
Know Your Study
Gain oversight into which sites are engaged with your study and what queries they have. Track whether communications are being read – or not. Use surveys to get direct site feedback.
Clinical trials operating at lightspeed
We use Teckro to drive the recruitment that we need and make sure that our recruitment will be met on time.
Clinical Research Staff & Investigators
Peace of Mind With Answers in Your Pocket
Research staff and investigators have critical study answers anytime, anywhere. Interconnect your digital protocol with secure communication channels in a simple mobile app. Enable the best-informed decisions at the point of care.

Reduce Your Tech Burden
Avoid the headache of manually tracking your many study system passwords. Seamlessly sign-in to study applications across multiple vendors using simple, secure QR code authentication.
Always the Right Version
Access the right protocol version approved for your site. Amendments assigned to you in a digital instant. Easily search all available study resources for the answers you need, in a tap.
Experts Within Reach
Never wonder who to call again with study contacts at your fingertips. Dedicated groups of experts available to guide and clarify, for informed patient decisions at the point of care.
Revolutionizing the CRC day job
It’s rare that I would speak to a sponsor as a coordinator, but it is imperative that sponsors get site feedback. Teckro allows sites to communicate in a way that they previously wouldn't have been able to, meaning sponsors can become aware of the real issues sites are having.

Grayson Scott
Site Supervisor at Centricity Research
Study Monitors
Cut Through the Noise with a Mobile Study HQ
Study monitors and CRAs have one place for study information and communication channels to simplify site support, even on the-go. Oversight without the bottleneck, monitors gain time for essential study tasks.
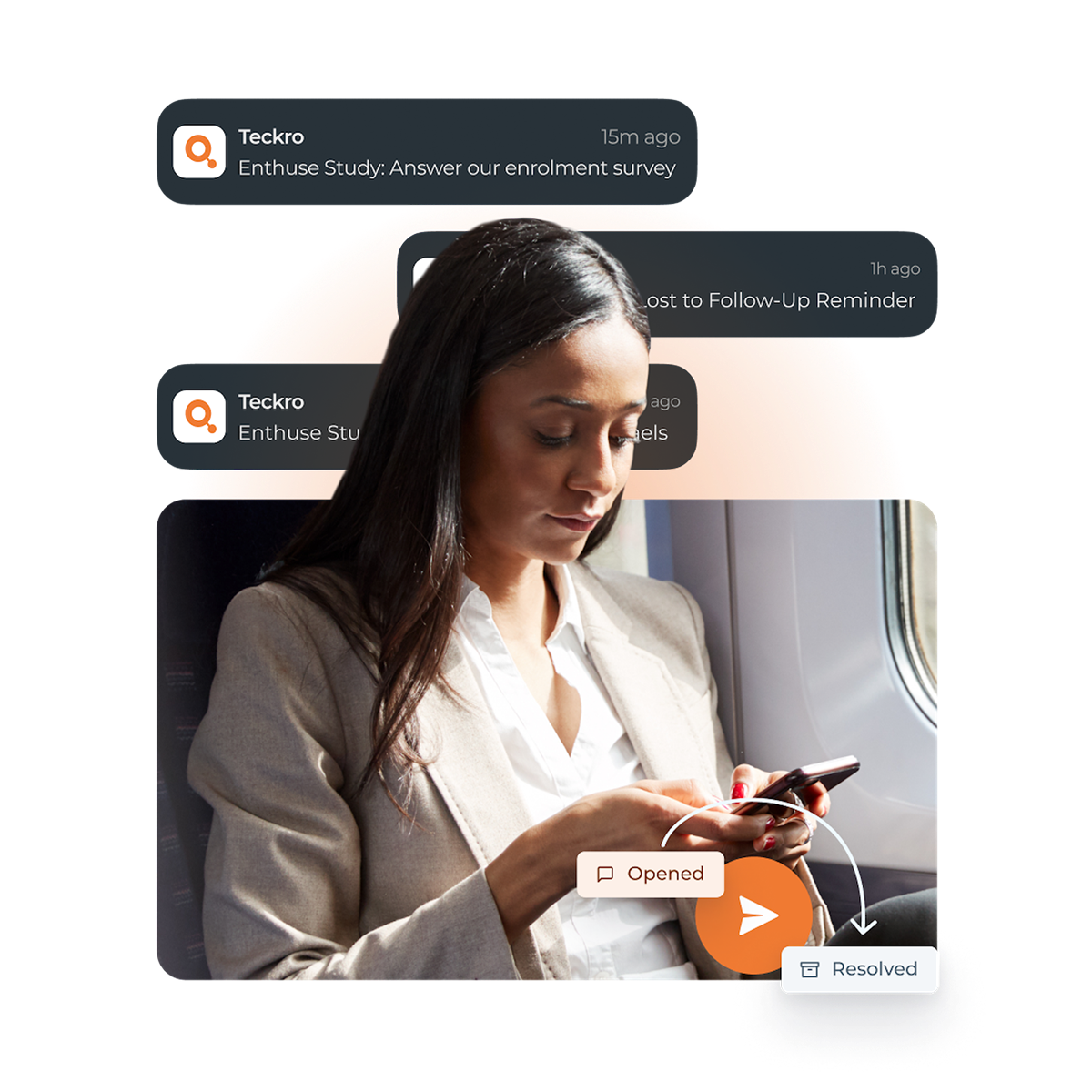
Keep Up with Changes
User changes. Document changes. Site changes. Simplify study management and stay ahead. Avoid missing updates with push notifications that cut through the avalanche of emails and calls.
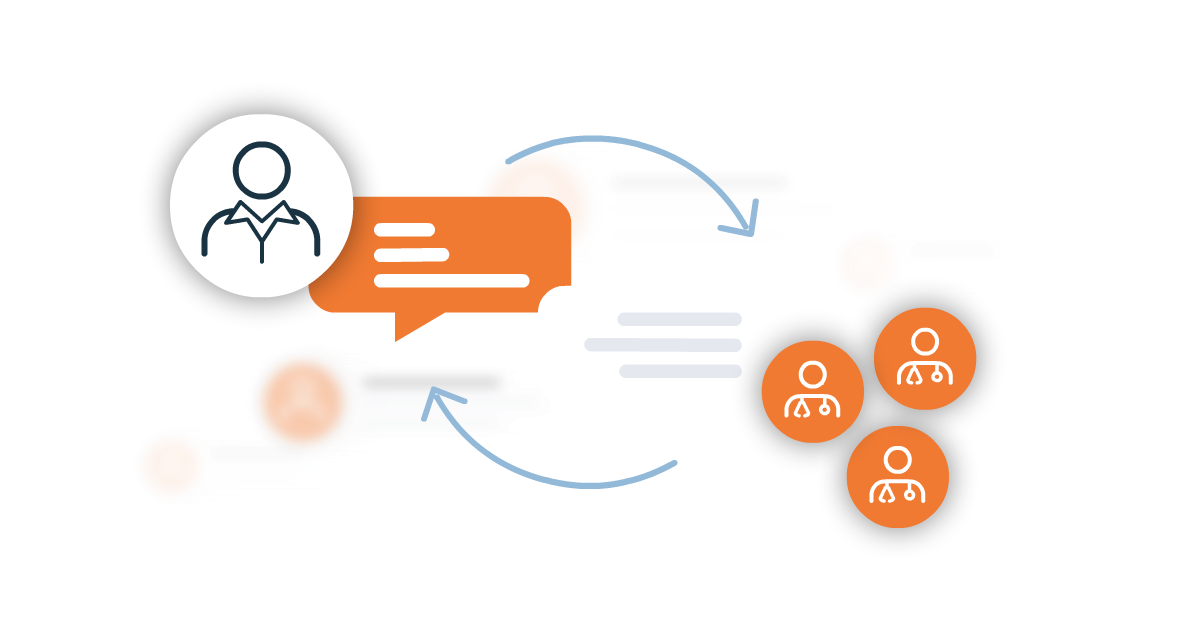
Streamline Communication
Consolidate site-sponsor communication. Less switchboard running and more study monitoring, helping support data integrity. Gain oversight and eliminate manual routing.
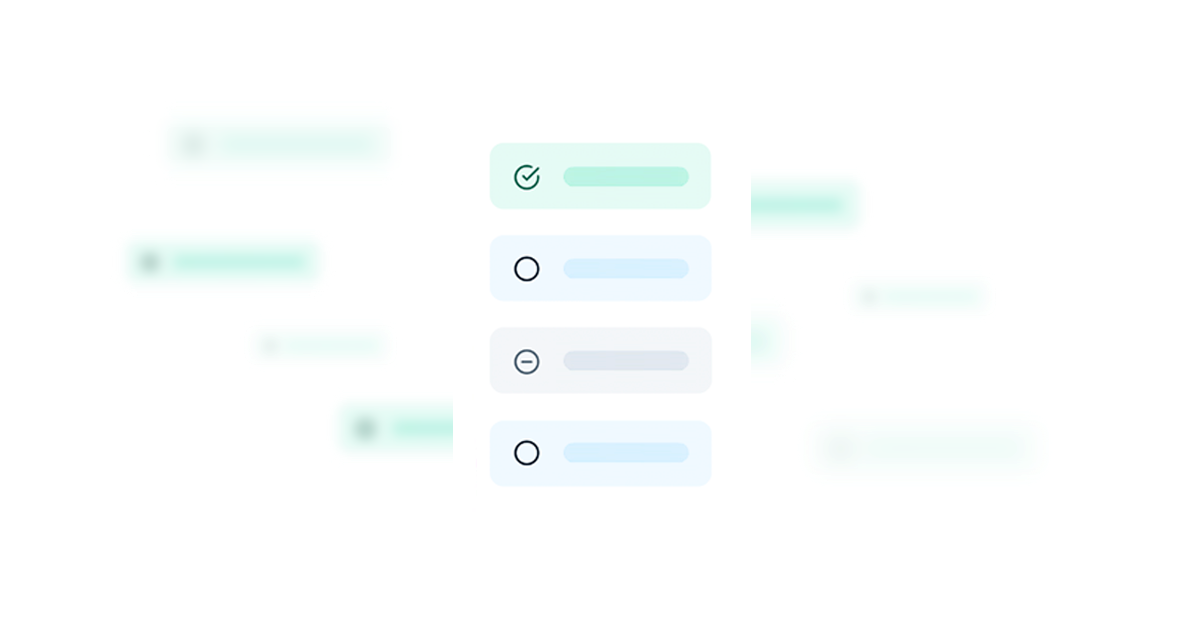
Less Stressful SIVs
Site activation tasks easily monitored in a central checklist. Automated workflows notify you of task completion. Progress is transparent. Trackers are a thing of the past.
Collaboration made simple - stop herding cats
I believe that if all studies had Teckro, sites would show a better adherence to study protocols as they often make mistakes just for not having the necessary documents with them.